Why Choose Onsior?
Convenient to administer in the hospital and at home.
A coxib NSAID that is rapidly absorbed, persists at the site of inflammation and is rapidly cleared from the bloodstream.*2,3
*Clinical relevance unknown.
Well-tolerated when administered at a dose of 1 mg/kg (tablets) once daily or at a dose of 2 mg/kg subcutaneously once daily to cats for up to a maximum of 3 days.
Effective at controlling the postoperative pain and inflammation associated with orthopedic surgery, ovariohysterectomy, and castration in cats
Onsior Product Information

Control of postoperative pain and inflammation

Orthopedic surgery, ovariohysterectomy, and castration in cats

Tablets, Injection

Robenacoxib
Onsior Dosing Table
Tablet Dosing Range
| 6 mg Tablets - 1 |
| Weight (lbs) - 5.5 to 13.2 lbs |
| Weight (kg) - 2.5 to 6 kg |
| 6 mg Tablets - 2 |
| Weight (lbs) - 13.3 to 26.4 lbs |
| Weight (kg) - 6.1 to 12 kg |
| 6 mg Tablets | Weight (lbs) | Weight (kg) |
|---|---|---|
| 1 | 5.5 to 13.2 lbs | 2.5 to 6 kg |
| 2 | 13.3 to 26.4 lbs | 6.1 to 12 kg |
Subcutaneous Injection Dosing
| Dose in Kilograms - 2 mg/kg |
| Dose in pounds - 0.91/lb |
| Dose in Kilograms | Dose in pounds |
|---|---|
| 2 mg/kg | 0.91/lb |
Administering Onsior for Surgery
| DAY 1 - Administer the first Onsior dose approximately 30 minutes before soft-tissue surgery. |
| DAY 2 - Administer (or instruct the owner to administer) the second Onsior dose approximately 24 hours after the first dose. |
| DAY 3 - Administer (or instruct the owner to administer) the third and last Onsior dose approximately 24 hours after the second dose. |
| DAY 1 | DAY 2 | DAY 3 |
|---|---|---|
| Administer the first Onsior dose approximately 30 minutes before soft-tissue surgery. | Administer (or instruct the owner to administer) the second Onsior dose approximately 24 hours after the first dose. | Administer (or instruct the owner to administer) the third and last Onsior dose approximately 24 hours after the second dose. |
How does Onsior work?
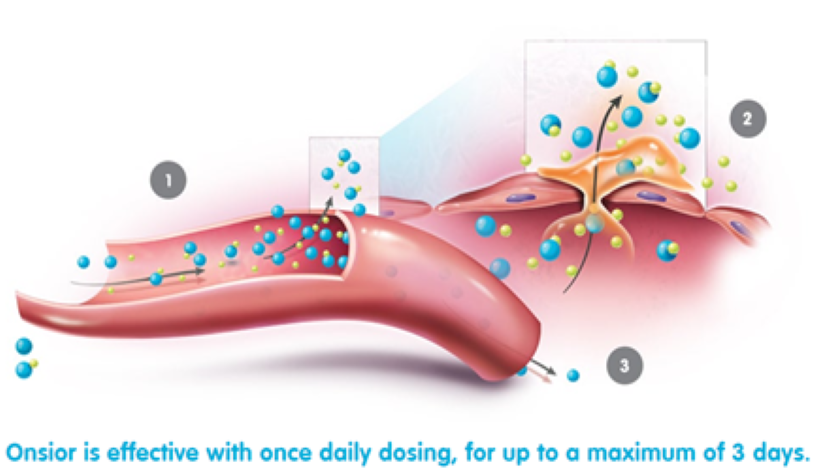
Onsior is quickly absorbed. Peak blood concentrations are attained rapidly with median Tmax values generally occurring within 0.5 hr.*
Onsior, a highly protein-bound NSAID, persists longer at the site of inflammation than in the blood because inflammatory exudate is rich in plasma proteins, thereby providing sustained drug levels at the site of action.2,3*

Onsior is rapidly eliminated from the blood. The mean blood T1/2 is approximately 1.7 hrs for Onsior Tablets for Cats.* The terminal T1/2 is approximately 1.1 hrs. for Onsior injection for Cats.***
* Tablets dosed at 1 mg/kg without food.
**Clinical relevance unknown.
*** Injection dosed at 2 mg/kg.
Onsior® (robenacoxib) Resources
Help get them from the hospital to home comfortably
To discover how you can incorporate Onsior into your surgical protocol today, contact your local Elanco rep, distributor rep or call Elanco at 1-888-545-5973.
Onsior® (robenacoxib) for Cats
Indications for Cats
ONSIOR® tablets for Cats are indicated for the control of postoperative pain and inflammation associated with orthopedic surgery, ovariohysterectomy and castration, in cats ≥ 5.5 lbs and ≥ 4 months of age; for up to a maximum of 3 days.
ONSIOR® injection is indicated for the control of postoperative pain and inflammation associated with orthopedic surgery, ovariohysterectomy and castration in cats ≥ 4 months of age; for up to a maximum of 3 days.
Important Safety Information for Cats
Do not use in cats that have hypersensitivity to robenacoxib or known intolerance to NSAIDs. Do not administer Onsior® tablets or injection in conjunction with any other NSAID or corticosteroid. Do not use for more than 3 days. A thorough history and physical exam including appropriate testing should be conducted before initiation of a NSAID therapy. Owners should be advised to observe for signs of potential drug toxicity. Stop administration of ONSIOR® if appetite decreases or if the cat becomes lethargic. The use of ONSIOR® has not been evaluated in cats used for breeding, or in pregnant or lactating cats or in cats with cardiac disease. As a class, cyclo-oxygenase inhibitory NSAIDS may be associated with gastrointestinal, renal, and hepatic toxicity. For full prescribing information see label links below.
ONSIOR® injection: For subcutaneous use in cats ≥ 4 months of age. Safety has not been demonstrated for intravenous or intramuscular administration. The most common adverse reactions were incision site infection, increased incision site bleeding, vomiting, inappetence and lethargy.
ONSIOR® tablets for Cats: For oral use in cats ≥ 5.5 lbs and ≥ 4 months of age only. The most common adverse events are anorexia, depression, vomiting, elevated BUN, elevated creatinine, and renal insufficiency/failure.













